Green chemistry is becoming one of the most popular and relevant branches of chemistry. This article highlights some of the amazing green chemistry research conducted at McMaster University. Dr. Brook (photo below) is a professor who helped found McMaster’s Sustainable Chemistry program. His work researches on increasing the sustainability of silicone chemistry.

Photo of Dr. Brook, Professor Emeritus, Faculty of Science Chair in Sustainable Silicone Polymers at McMaster University.
Silicon is an element on the periodic table that has many purposes commonly ingrained into our daily lives. It is the primary element of silicone, a polymer often used in adhesives and coatings. The silicone polymer is made up of repeating siloxane bonds, which are bonds between silicon and oxygen atoms.
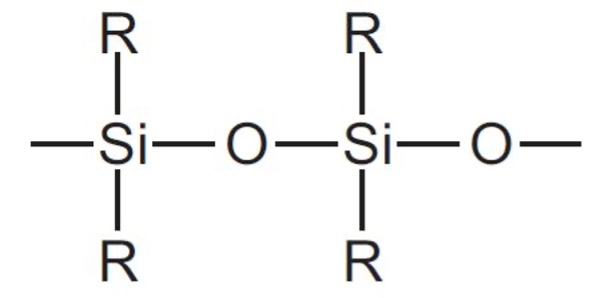
Figure 1. A siloxane bond is characterized by a silicon atom bound to an oxygen atom.
Materials made off silicone have unique mechanical properties due to their elasticity. These properties allow silicones to be used in many energy-save uses, like car tire rubbers. Since silicone oils can be environmentally degraded using water, carbon dioxide and sand (a natural silicone), they show potential for sustainable uses. Nonetheless, creating silicones from sand is a highly energetic process. Certain processes in the creation of repeating siloxane units can use fossil fuels, leading to greenhouse gas production. Simply put, the more siloxane bonds in a material, the more energy the silicone’s creation needs. Thus, the Brook Lab aimed to remove this costly process and apply green chemistry principles to silicone creation.
Simple silicones only contain repeating units of silicon-oxygen bonds. But, this leads to a higher density of silicon than need to fulfill its purpose. The result is increased levels of energy needed to form a single polymer. But what if there existed a renewable compound that spaced out siloxane bonds and reduced energy needs?
Researchers Chen et al. chose to investigate starch for this purpose. Starch is commonly found in foods like potatoes but less commonly in chemistry labs. The team discovered that starch successfully meshed into a network of siloxane bonds (Figure 2). This spaced out the high-energy bonds, leading to decreased density and energy needed to form the polymer. This leads to an important question: can starch-silicone compounds keep the properties that make traditional silicones useful?
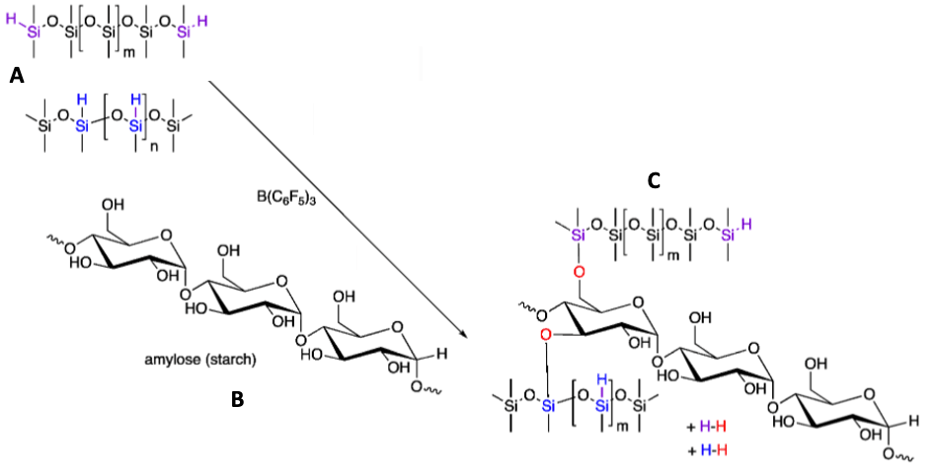
Figure 2. The chemical reaction scheme shows silicone (A) being merged with starch (B) to create starch-silicone polymers (C).
After lengthy research, Chen et al. found promising results. The team discovered that though to a lesser extent, the starch-silicones replicate many desirable elastic properties of traditional silicones. Also, the physical properties of the silicone could be manipulated depending on starch concentration. The rigid elastomer can be altered to a softer gel. Thus, this alternative method has potential.
These starch-silicones are lower quality but can replace up to 75% of silicones needed while maintaining essential elastic properties. This introduces a fantastic lower-energy option for applications that only need mild elasticity.
The Brook Lab successfully created a more sustainable silicone that can replace some cases of traditional silicone. Though the approach is still juvenile, research can extend applicability to replace more silicones in our daily lives.
The finding of this work has been published in Sustainability: Chen, Y.; Valentini, D. A.; Brook, M. A. Starch/Silicone Elastomers and Foams. Sustainability. 2023, 15 (13), 9941. https://doi.org/10.3390/su15139941.
About the Author

MUSCS
The McMaster Undergraduate Society for Chemical Sciences (MUSCS) is a student-run organization dedicated to enhancing the undergraduate experience for all McMaster University Chemistry & Chemical Biology Students. You can check out their Instagram page here.
